
Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse kidney tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing Nicotinic Acetylcholine Receptor (MA3-043) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
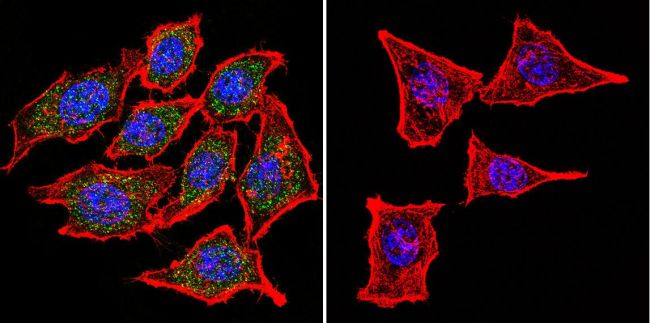
Immunofluorescent analysis of Nicotinic Acetylcholine Receptor using Anti-Nicotinic Acetylcholine Receptor Monoclonal Antibody (88B) (Product# MA3-043) shows staining in Hela Cells. Nicotinic Acetylcholine Receptor staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Nicotinic Acetylcholine Receptor (Product# MA3-043) at a dilution of 1:100 over night at 4 ◦C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product# 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
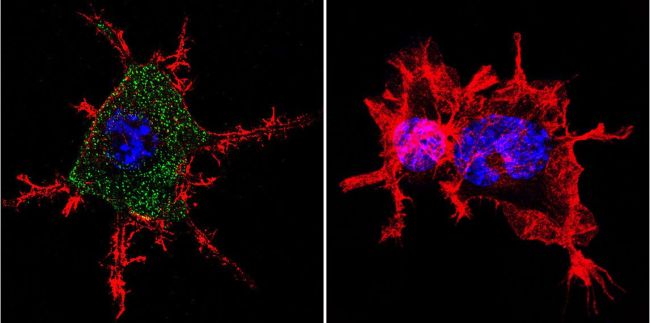
Immunofluorescent analysis of Nicotinic Acetylcholine Receptor using Anti-Nicotinic Acetylcholine Receptor Monoclonal Antibody (88B) (Product# MA3-043) shows staining in Neuro-2a Cells. Nicotinic Acetylcholine Receptor staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Nicotinic Acetylcholine Receptor (Product# MA3-043) at a dilution of 1:100 over night at 4 ◦C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product# 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
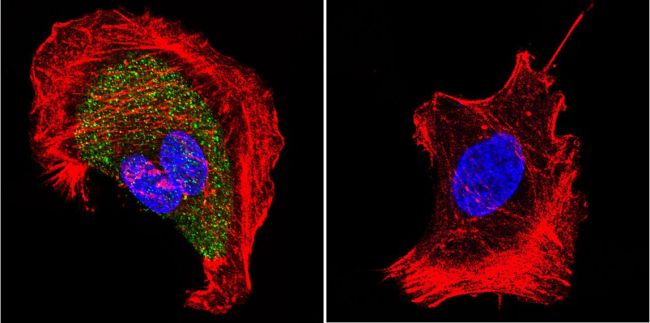
Immunofluorescent analysis of Nicotinic Acetylcholine Receptor using Anti-Nicotinic Acetylcholine Receptor Monoclonal Antibody (88B) (Product# MA3-043) shows staining in U251 Cells. Nicotinic Acetylcholine Receptor staining (green), F-Actin staining with Phalloidin (red) and nuclei with DAPI (blue) is shown. Cells were grown on chamber slides and fixed with formaldehyde prior to staining. Cells were probed without (control) or with or an antibody recognizing Nicotinic Acetylcholine Receptor (Product# MA3-043) at a dilution of 1:20 over night at 4 ◦C, washed with PBS and incubated with a DyLight-488 conjugated secondary antibody (Product# 35503, Goat Anti-Mouse). Images were taken at 60X magnification.
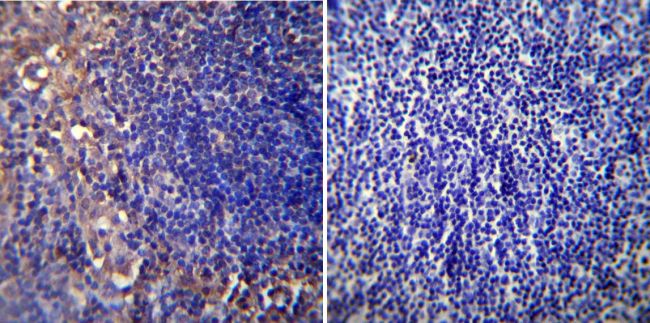
Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse lymph node tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing Nicotinic Acetylcholine Receptor (MA3-043) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.
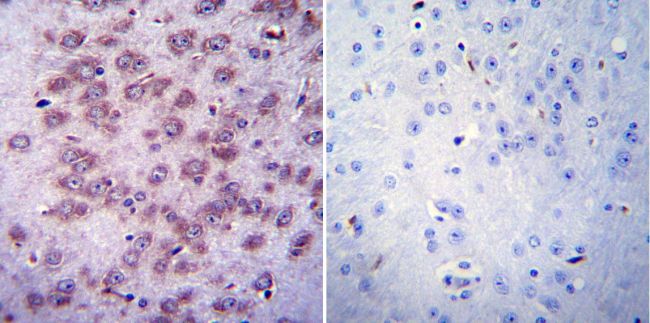
Immunohistochemistry was performed on normal biopsies of deparaffinized Mouse brain tissue. To expose target proteins, heat induced antigen retrieval was performed using 10mM sodium citrate (pH6.0) buffer, microwaved for 8-15 minutes. Following antigen retrieval tissues were blocked in 3% BSA-PBS for 30 minutes at room temperature. Tissues were then probed at a dilution of 1:20 with a mouse monoclonal antibody recognizing Nicotinic Acetylcholine Receptor (MA3-043) or without primary antibody (negative control) overnight at 4°C in a humidified chamber. Tissues were washed extensively with PBST and endogenous peroxidase activity was quenched with a peroxidase suppressor. Detection was performed using a biotin-conjugated secondary antibody and SA-HRP, followed by colorimetric detection using DAB. Tissues were counterstained with hematoxylin and prepped for mounting.





